Mobility Database
PanAl2024_MB is an atomic mobility database for Al-based alloys, which is compatible with the PanAl2024_TH thermodynamic database and suitable for the simulation of diffusion-controlled phenomena using the PanDiffusion module, PanEvolution module, and/or PanSolidification module.
Phases
The atomic mobility within the Liquid, Bcc, Fcc, and Hcp solution phases are assessed in this database.
Self-diffusivity of Pure Elements
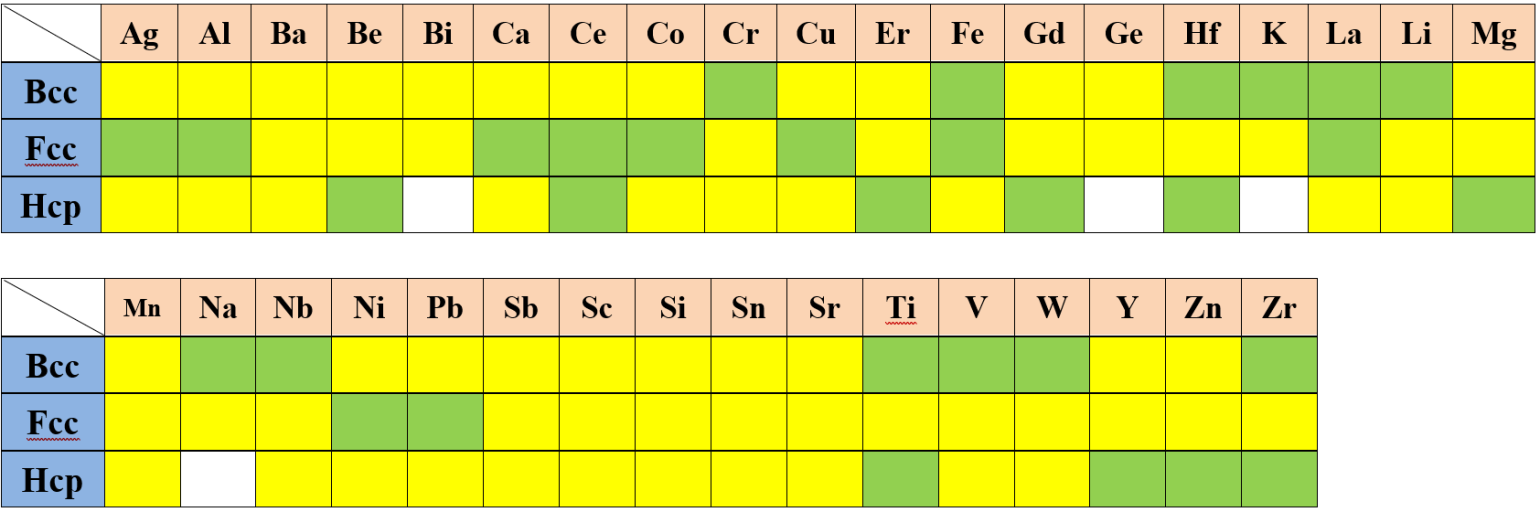
The self-diffusivity of an element is usually described by an analytical expression. For the stable crystal structures, these expressions can be obtained using the available experimental data, while those for the metastable/unstable states are usually estimated from those of the stable states. In the following tables, we use different color to represent different status:
| : | Validated | |
| : | Estimated | |
| : | No data |
Assessed Systems
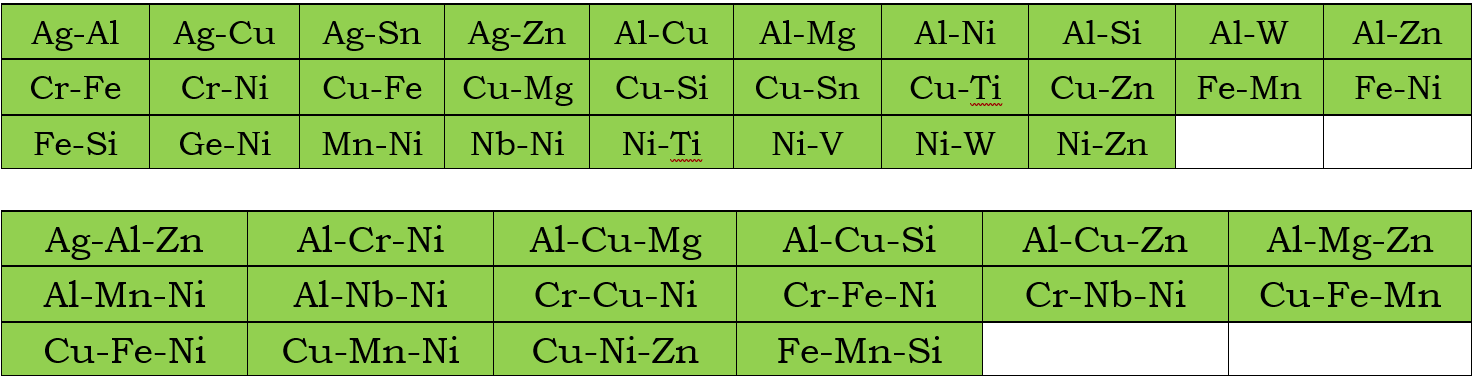
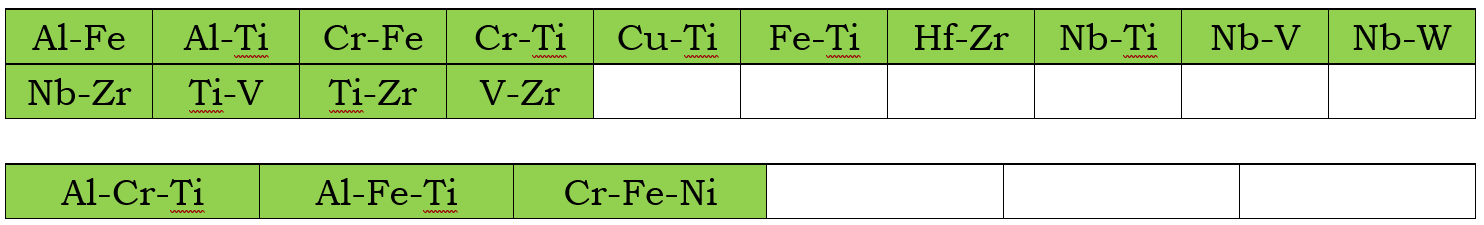
In addition to the assessed self-diffusivities shown above, the impurity diffusion data for all elements included in the current mobility database are also assessed. Moreover, chemical-diffusivities available in some binary and ternary systems are also used to assess the interaction parameters. These binary and ternary systems are listed below for the Bcc, Fcc, and Hcp phases, respectively.
Fcc Phase
Bcc phase
Database Validation
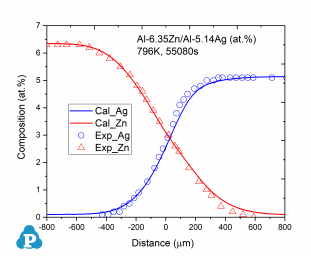
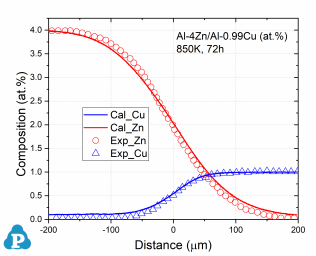
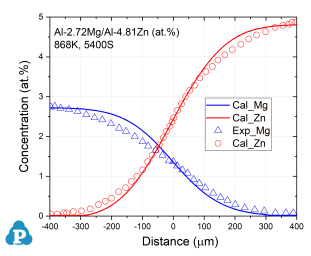
The simulated concentration profiles of a series of aluminum alloys are used to validate the current mobility database for Al-based alloys. A few examples of such simulation are shown below.
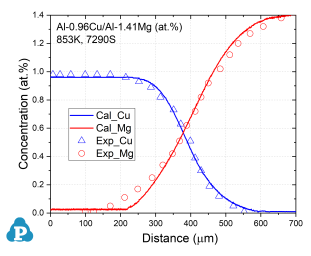
Figure 3: Concentration profiles of Al-0.96Cu/Al-1.41Mg (at.%) aged at 853K for 7290s [2010Zha, 2014Xin]
Applications
This mobility database is combined with the thermodynamic database for Al-based alloys, PanAl_TH, to simulate the diffusion-controlled phenomena of Al-based alloys. A few examples are given below.
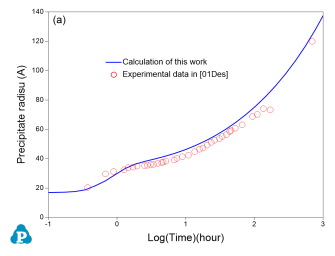
Precipitation kinetics of aluminum alloys
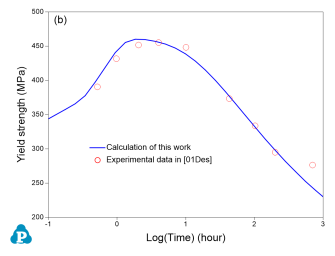
The PanEvolution module was developed for the simulation of precipitation kinetics of multi-component alloys. It has been seamlessly integrated with the thermodynamic calculation engine of Pandat™ software, and has been used to simulate the evolution of microstructure and the corresponding mechanical property responses to heat treatment of 2xxx, 6xxx and 7xxx series of aluminum alloys [2011Cao]. Below shows an example simulation performed for the Al-2.3Mg-6.1Zn (wt%) alloy aged at 160 °C for 1000 hours. The simulated particle size and yield strength evolution with time are compared with experimental data as shown in Figure 5
Figure 5: Simulated and measured particle size and yield strength evolution with time for Al-2.3Mg-6.1Zn (wt%) alloy aged at 160 °C for 1000 hours
As is seen, the particles grow and coarsen with ageing time, while the yield strength reaches peak between 1 to 10 hours. The yield strength decreases quickly after 10 hours of ageing at 160 °C. The database used to do this simulation is the combined thermodynamic and mobility database of Al-based alloys: PanAl_TH+MB. More information regarding to precipitation simulation can be found in PanEvolution module under the Software section.
Dissolution of aluminum alloys
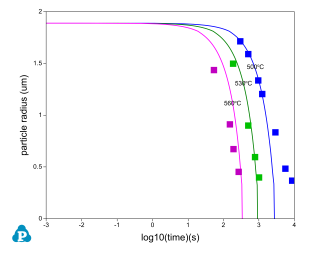
The PanDiffusion module was developed for the simulation of diffusion kinetics of multi-component alloys. In Figure 6, dissolution of Si particle in Al-Si binary system was simulated and compared with the experimentally determined data [1992Tun]. In this simulation, the combined thermodynamic and mobility database of Al-based alloys, PanAl_TH+MB, is used. More information regarding to diffusion simulation can be found in PanDiffusion module under the Software section.
Figure 6: Comparison of simulated and experimentally determined dissolution of Si particle in Al-Si binary system
Solidification of aluminum alloys
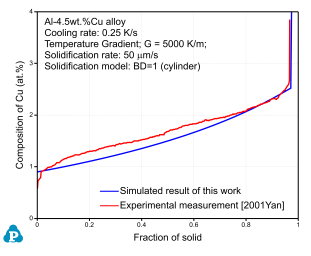
The PanSolidification module was developed for the simulation of solidification behavior of multi-component alloys considering the effects of back-diffusion in the solid matrix phase, cooling rate, and dendrite arm coarsening. As shown in Figure 7, the solidification of the Al-4.5wt.%Cu alloy at the cooling rate of 0.25K/s is simulated and compared with the experimentally determined data [2001Yan].
Figure 7: Comparison of simulated and experimentally determined Cu composition profile within the Fcc matrix for the Al-4.5wt.%Cu alloy at the cooling rate of 0.25K/s
In this simulation, the combined thermodynamic and mobility database of Al-based alloys, PanAl_TH+MB, is used. More information regading to solidification can be found in PanSolidification module under the Software section.
[1992Tun] U.H. Tundal and N. Ryum, Dissolution of particles in binary alloys: Part II. experimenal investigation on an Al-Si alloy. Metallurgical and Materials Transactions A, 23 (1992): 445-449.
[2001Yan] X. Yan, Thermodynamic and Solidification Modeling Coupled with Experimental Investigation of the Multicomponent Aluminum Alloys, in Materials Science and Engineering. (2001), University of Wisconsin-Madison: Madison, WI.
[2008Yao] J.J. Yao, et al., Diffusional mobility for fcc phase of Al-Mg-Zn system and its applications. Calphad, 32 (2008): 602-607.
[2010Cha] H. Chang, et al., Assessment of the atomic mobilities for ternary Al-Cu-Zn fcc alloys. Calphad, 34 (2010): 68-74.
[2010Zha] W.B. Zhang, et al., Assessment of the atomic mobility in fcc Al-Cu-Mg alloys. Calphad, 34 (2010): 286-293.
[2011Cui] S.L. Cui, et al., Assessment of Atomic Mobilities in fcc Al-Ag-Zn Alloys. Journal of Phase Equilibria and Diffusion, 32(6) (2011): 512-524.
[2011Cao] W. Cao, et al., An Integrated Computational Tool for Precipitation Simulation. JOM, 63(7) (2011): 29-34.
[2014Xin] J.H. Xin, et al., Prediction of diffusivities in fcc phase of the Al-Cu-Mg system: First-principles calculations coupled with CALPHAD technique. Computational Materials Science, 90 (2014): 32-43.